Responsible Conduct of Research Policy and Training Plan
Approved by: Provost
Date Established:
Responsible Office: Office of the Provost
Date Last Revised: Sept. 30, 2025
Responsible Administrator: Director of Faculty Research and Grants
Purpose Statement
The purpose of this policy is for Kalamazoo College to maintain compliance with both state and federal regulations and guidelines aimed at ensuring integrity and accountability in research practices. In addition, to fulfill funding requirements for RCR training for Designated Researchers as mandated by federal agencies such as the National Institutes of Health (NIH) and the National Science Foundation (NSF).
Ethical and responsible conduct of research is critical for excellence, as well as public trust. Consequently, education in the responsible and ethical conduct of research is considered essential in the preparation of future researchers. The NSF, the primary source of Kalamazoo College’s federal funding, advocates a similar philosophy: “The Responsible and Ethical Conduct of Research (RECR) is critical for excellence, as well as public trust, in science and engineering.” Similarly, the NIH holds as a core principle the belief that “responsible conduct of research is a fundamental element of research training.”
A campus-wide environment pertaining to the responsible and ethical conduct of research requires researchers to have the knowledge, skills, and tools to enable them to be responsible for their research conduct. A basic understanding of the responsible conduct of research can be obtained and documented through Kalamazoo College’s affiliation with the Collaborative Institutional Training Institute (CITI) Program (https://www.citiprogram.org/). Kalamazoo College offers its faculty, staff, and students free access to CITI’s various topic areas and corresponding certifications that hold widely recognized value within the research space. For students, obtaining CITI certification is an excellent indicator to future employers that they have knowledge of research ethics and have been involved in substantive scientific work.
In compliance with the National Science Foundation’s (NSF) implementation of Section 7009 of the America COMPETES Act (42 U.S.C. 1862o-1), all Designated Researchers supported by NSF to conduct research are required to complete training in the responsible and ethical conduct of research. This applies to proposals submitted on or after July 31st, 2023 and subsequently issued as an award. NSF incorporates the Responsible Conduct of Research (RCR) requirement into the terms and conditions of applicable awards.
Definitions
These definitions apply to terms as they are used in this policy.
Term Definition
Designated Researcher All undergraduate students, post-baccalaureate fellows, graduate students, postdoctoral fellows, faculty members, senior personnel, and staff members conducting research.
Policy Statement
Responsible Conduct of Research training is required of all Designated Researchers prior to conducting human subjects research, animal subjects research, and/or engaging in NSF- or NIH-funded research. There is an additional research security training requirement for all named grant personnel which must be completed in advance of any grant submissions to NIH and NSF.
Policy Violations
Violations of College policies are adjudicated according to procedures outlined in the Student Handbook and the Employee Handbook, with disciplinary consequences imposed by the adjudicating authority up to and including dismissal. Some offenses are punishable under state and federal laws.
Scope/Responsibilities
Responsibilities:
- For the following policy: All persons who are engaged in research at Kalamazoo College
- For enforcement and oversight of policy: Office of the Provost, Director of Faculty Research and Grants
- For procedures implementing policy: IRB Committee; OSHA Director; and Faculty Grants Office.
Principal investigators and/or project directors who conduct research are responsible for ensuring that undergraduate students, post-baccalaureate fellows, graduate students, postdoctoral researchers, faculty members, and senior/key personnel who participate in their research complete RCR training as needed according to this plan. Principal investigators are also responsible for completing RCR training appropriate to their career stage and as outlined in sponsored research award requirements.
Principal investigators are responsible for completion of the required RCR training and maintaining training documentation of RCR training for all researchers who work under their direction. Principal investigators are, themselves, responsible for completing ongoing RCR training appropriate to their own career stage and subject matter and for maintaining documentation of this training. For principal investigators conducting externally sponsored research projects, this requires completion of any specific funding agency requirements, as applicable, and may include periodic refresher training.
Principal investigators submitting grants to Public Health Service (PHS) (which includes NIH, FDA, CDC, HRSA, AHRQ, and SAMHSA), NSF, or other funding agencies with RCR requirements, must confirm that they have reviewed and will comply with the College’s RCR policy and training plan during the pre-submission internal approval process, which is administered and certified by the Office of Faculty Grants. The internal routing process must be completed before principal investigators’ proposals can be submitted to the funding agency for review.
Procedures
The Kalamazoo College CITI Program offers numerous online courses focused on different areas of responsible conduct of research, including: human subjects (Institutional Review Board (IRB) Researchers), animal subjects (Working with the Institutional Animal Care and Use (IACUC), Working with mice in research settings, Working with zebrafish in research settings, Working with amphibians in research, Post-procedure care of mice and rats in research), financial conflicts of interest (Public Health Services Financial Conflict of Interest (PHS FCOI)), and funder-specific (Responsible and Ethical Conduct of Research (RECR for NSF)) courses.
Procedure for Compliance:
RCR training is required for any Designated Researcher before conducting research involving human subjects, regardless of the discipline. This includes secondary data analysis, unless the dataset has been designated by IRB as not human subjects research due to de-identification.
RCR training focused on animal welfare in terms of ethical principles and federal regulations is required for any Designated Researcher before conducting research involving live vertebrate animals or live cephalopods. The minimum training required includes completion of the CITI Program course, “Working with the IACUC: Animal Investigators, Staff and Students.”
RCR training is additionally required for any Designated Researcher conducting research in any discipline who are paid through NSF grants that are administered by the College. Principal investigators paid through NIH or other PHS agencies are required to complete in-person RCR training for specific award types (see RCR Guidelines below). This RCR requirement also applies to anyone paid through grants from other funders that require RCR training per the associated funding announcement and/or funding agency guidelines.
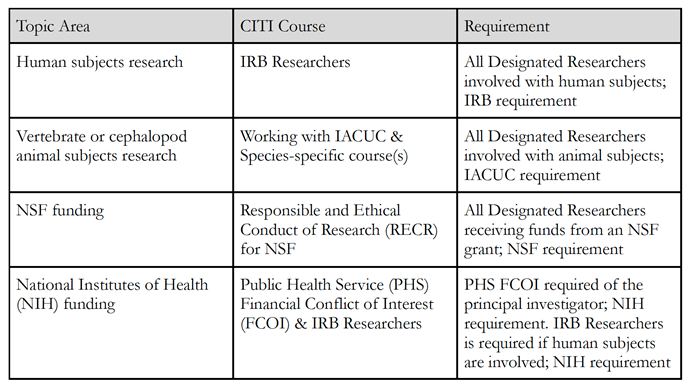
Specific Funding Agencies
Principal investigators who receive grant funding from PHS or NSF are responsible for complying with Kalamazoo College’s RCR Policy and Training Plan, as well as any additional requirements from the funding agency. Training must be completed within 60 days (or sooner, if stipulated by the funder) of initial engagement in the sponsored research activity for Kalamazoo College to remain compliant with federal requirements. Guidance for complying with PHS and NSF RCR training requirements is provided below. Investigators should contact the Facutly Grants Office with any questions pertaining to specific funding agency requirements.
Principal investigators should note that all Kalamazoo College CITI RCR training courses are valid for four years (with the NSF RCR training course as the one exception), after which point a refresher course is required. CITI account holders will receive an email notice in advance of course expiration.
Guidelines
RCR training is recommended for all undergraduate students, post-baccalaureate fellows, graduate students, postdoctoral fellows, faculty members, senior personnel, and staff members in research-adjacent disciplines, such as astronomy, biochemistry, biological sciences, chemistry, computer science, engineering, education & child study, environmental science & policy, geosciences, marine science & policy, mathematics, neuroscience, physics, psychology, and statistical & data sciences.
Documentation and Compliance
The Director of Faculty Research and Grants tracks compliance with the RCR Policy and Training Plan for all principal investigators and researchers participating in externally sponsored projects, according to specific funding agency requirements. IRB provide RCR oversight for all projects that include human subjects and animals, respectively.
All externally sponsored principal investigators will work with the Faculty Grants Office to comply with sponsor-required RCR training during both grant submission and award set-up. Additionally, principal investigators will work with the Faculty Grants Office to comply with sponsor-required RCR training when adding any undergraduates or postdoctoral researchers to their externally sponsored projects.
Externally Sponsored Research Training Requirements
NSF
For NSF proposals submitted on or after July 31, 2023, all faculty and other senior personnel named on the proposal are required to complete the CITI program’s NSF RCR training before any funding can be awarded. Undergraduates and postdoctoral researchers are required to complete NSF RCR training before participating in any NSF-supported research.
Research Security Requirements
There is an additional research security training course required for all named grant personnel (e.g., PI, co-I, other senior/key personnel). It is provided through Kalamazoo College’s Moodle system and must be completed 12 months prior to any NSF grant submissions.
Institutions are responsible for reviewing investigators’ Significant Financial Interest. All investigators must complete a form submitted to the Faculty Grants Office as part of the internal routing process. It is recommended that investigators complete CITI’s Financial Conflict of Interest training during award set-up and in advance of sponsored research activity or have completed the FCOI certification in the last 4 years.
DHHS/PHS (NIH, AHRQ, HRSA, CDC, FDA, SAMHSA)
All trainees, fellows, participants, and scholars receiving support through any NIH training grant, career development award (individual or institutional), research education grant, or dissertation research grant must receive instruction in RCR.
Existing NIH policy calls for RCR instruction to be undertaken at least once during each career stage, and at a frequency of no less than once every four years. More than 50% of RCR training for PHS-funded investigators must take place in-person; CITI training can be used for some of the hours but not for the majority. Acceptable programs generally involve at least eight contact hours.
These guidelines apply specifically to NIH’s D43, D71, F05, F30, F31, F32, F33, F34, F37, F38, K01, K02, K05, K07, K08, K12, K18, K22, K23, K24, K25, K26, K30, K99/R00, KL1, KL2, R25, R36, T15, T32, T34, T35, T36, T37, T90/R90, TL1, TU2, and U2R programs. This policy also applies to any other NIH-funded programs supporting research training, career development, or research education that require instruction in responsible conduct of research as stated in the relevant funding opportunity announcements.
Institutions are responsible for reviewing investigators’ Significant Financial Interest. All investigators must complete a form submitted to the Faculty Grant Office as part of the internal routing process. Investigators are required to complete CITI’s Financial Conflict of Interest training during award set-up and in advance of sponsored research activity or have completed the FCOI certification in the last 4 years.
Research Security requirements
There is an additional research security training course required for all named grant personnel. It is provided through Kalamazoo College’s Moodle system. All Kalamazoo College representatives who are named NIH grant personnel (e.g., PI, co-I, other senior/key personnel) must complete this research security training on an annual basis.
If considered a clinical trial by NIH (human subjects, prospectively assigned, influencing outcomes), everyone involved with human subjects on the study directly or through data analysis is required to complete a Good Clinical Practices (GCP) training. This course is available online through the CITI Program or the Society of Behavioral Medicine; many research-focused conferences offer GCP training as an additional workshop.
